 Funding: European Union, Horizon 2020 MSCA Innovative Training Network (ITN) (Grant Agreement 813869)
Funding: European Union, Horizon 2020 MSCA Innovative Training Network (ITN) (Grant Agreement 813869)
Partners: National University of Ireland, Galway (Project Coordinator), IMDEA Materials Institute, Boston Scientific Limited, The Queens University of Belfast (UK), RWTH Aachen (DE), Vascular Flow Technologies Ltd. (UK), Meotec GmbH (DE), ITA TextilTechnologieTransfer GmbH (DE)
Project period: 2019 – 2022
Project Coordinador: Dr. Ted Vaughan (ted.vaughan@nuigalway.ie); at IMDEA: Prof. Javier Llorca (javier.llorca@imdea.org)
Project Website: https://bioimplantitn.eu/
BioImplant Innovative Training Network (ITN) is an ambitious European Industrial Doctorate programme that will provide world-class multidisciplinary training to 12 Early-Stage Researchers (ESRs) in the area of bioabsorbable medical implant development.
The research vision of the BioImplant ITN is to use an integrative approach that combines polymer-, metal- and ceramic-based bioabsorbable materials, to deliver functionally superior bioabsorbable materials with enhanced mechanical behaviour and controllable degradation profiles. The successful development of these materials has the potential to form the basis for the next-generation of medical implants.
Each of the 12 ESR projects implementing the research programme contains significant scientific and technological originality including innovative material processing strategies, polymer fibre- and textile-based materials, novel hybrid and composite material development, new computational modelling developments and new surface treatment developments to address specific gaps in current biomaterial capabilities.
The BioImplant ITN will also deliver a structured training programme that will enable the ESR community to innovate, develop and translate implant technologies from a clinical need to a commercial product. This integrated training programme will place research excellence at its core with advanced technical skills through hands-on research and structured training courses provided by the consortium network. The ESR community will also gain international and intersectoral experience through planned industrial placements and secondments, enhancing career development and employability and promoting their development into leading innovators in the European medical technology sector.
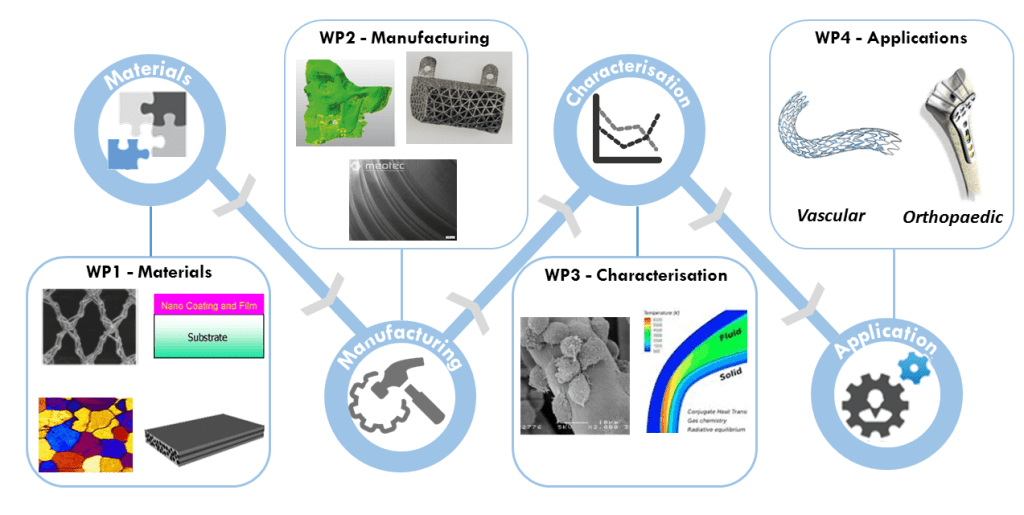
The scientific objective of the BioImplant ITN is to develop and implement improved bioabsorbable materials for vascular and orthopaedic implant applications. This next-generation of medical implants will be realised through technological innovation throughout the Supply Value Chain, including novel material development, advanced manufacturing technologies, robust characterisation and predictive capabilities and innovative application design. The specific research objectives of the BioImplant ITN are as follows:
- Enhance the mechanical properties of polymer-based bioabsorbables through novel processing technologies.
- Control degradation rates of magnesium-based bioabsorbable materials through innovative polymer and ceramic coating technologies.
- Develop novel metal- and ceramic-based polymer composite bioabsorbables that exhibit superior mechanical properties to traditional polymers using advanced manufacturing technologies.
- Predict degradation and long-term mechanical performance of implanted bioabsorbable devices using an integrated multiscale and multiphysics predictive modelling framework.
- Design, prototype and functionally test a series of small- and large-scale bioabsorbable implants for vascular and orthopaedic applications.
Currently, the Medical Technology sector employs over 650,000 people across the EU and files more patent applications with the European Patent Office than any other technical field. It is expected that the continuing innovation and development within this emerging sector will lead to extensive job opportunities across the EU for all ESRs. The technical expertise, transferrable skills and international experience the BioImplant ITN will provide to the ESR community will make them highly attractive to potential employers across academic, industry, regulatory and clinical settings in the Medical Technology sector.
Partners








Funded by


